Intramolecular Bonds exist inside molecules, for example Ionic and Covalent bonds.
Intermolecular Bonds exist between molecules. The stronger the intermolecular bonds the higher the boiling point. Two types of intermolecular bonds are Van der Waals bonds and Hydrogen bonds
Intermolecular Bonds exist between molecules. The stronger the intermolecular bonds the higher the boiling point. Two types of intermolecular bonds are Van der Waals bonds and Hydrogen bonds
A. Van der Waals bonds are based on electron distribution. Van de Waals bonds can be categorized in two categories: a weak bond created by the London Dispersion Force (LDF) or dipole-dipole bonds.
1. London Dispersion Forces (LDF)
These are the weakest intermolecular bonds. London Dispersion Forces are present in every molecule and are caused by the random movements of electrons inside atoms. Sometimes a large of electrons congregates on one side of an atom, causing a temporary dipole. The more electrons in the molecule the stronger the LDF.
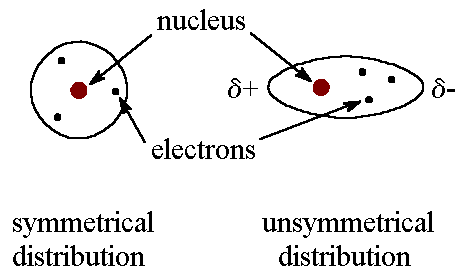
2. Dipole-Dipole Bonds
These exist only in polar molecules, where the negative and positive ends of molecules are attracted to the negative and positive ends of other molecules. These are stronger than London Dispersion Forces but weaker than Hydrogen Bonds.

A Polar Molecule
B. Hydrogen Bonds
When Hydrogen bonds with certain elements( Oxygen, Fluorine, Nitrogen, and in some cases Chlorine). Hydrogen Bonds are very strong and highly polar.

A Hydrogen Bond
These are the weakest intermolecular bonds. London Dispersion Forces are present in every molecule and are caused by the random movements of electrons inside atoms. Sometimes a large of electrons congregates on one side of an atom, causing a temporary dipole. The more electrons in the molecule the stronger the LDF.

2. Dipole-Dipole Bonds
These exist only in polar molecules, where the negative and positive ends of molecules are attracted to the negative and positive ends of other molecules. These are stronger than London Dispersion Forces but weaker than Hydrogen Bonds.

A Polar Molecule
B. Hydrogen Bonds
When Hydrogen bonds with certain elements( Oxygen, Fluorine, Nitrogen, and in some cases Chlorine). Hydrogen Bonds are very strong and highly polar.

A Hydrogen Bond
-Ben Suratos








